Supporting Provider and Patient Access
to Alcon's Innovative
Technologies and Medications

Alcon IOLs

Coding and
Billing Guides

Patient Rx
Affordability

Value Tool
Resources

Local
Support
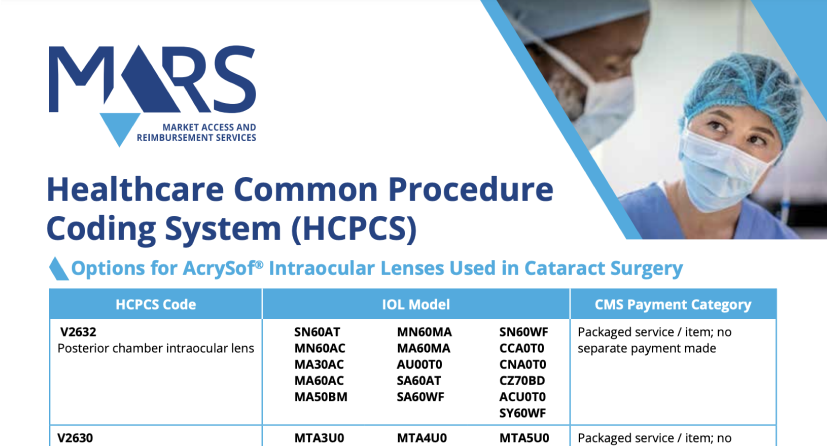
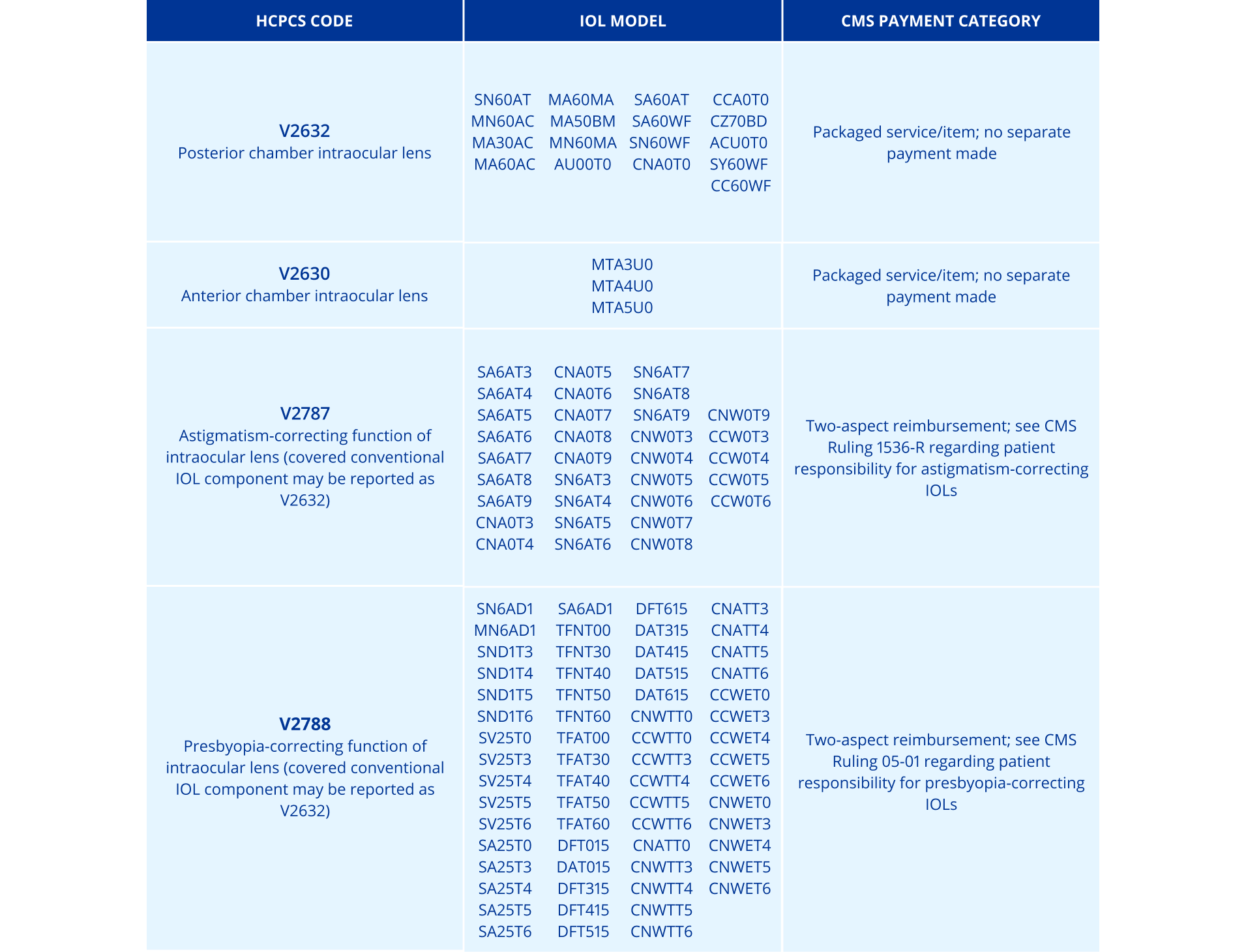
HCPCS Codes for Alcon Intraocular Lenses (IOLs)
Use the chart below to quickly find your HCPCS code for billing and reimbursement.
Important Product Information
Important Product Information - AcrySof® Family of IOLs
CAUTION: Federal law restricts these devices to sale by or on the order of a physician.
INDICATION: The family of AcrySof® single-piece intraocular lenses (IOLs) includes AcrySof® UV-absorbing IOLs (“AcrySof® UV”), AcrySof® IQ, AcrySof® IQ Toric, AcrySof® IQ ReSTOR®, AcrySof® IQ ReSTOR® Toric, AcrySof® IQ PanOptix®, AcrySof® IQ PanOptix® Toric, AcrySof® IQ Vivity™ and AcrySof® IQ Vivity™ Toric IOLs. Each of these IOLs is indicated for visual correction of aphakia in adult patients following cataract surgery. In addition, the AcrySof Toric IOLs are indicated to correct pre-existing corneal astigmatism at the time of cataract surgery. The AcrySof IQ ReSTOR IOLs are for cataract patients with or without presbyopia, who desire increased spectacle independence with a multifocal vision. The AcrySof® IQ PanOptix® lens mitigates the effects of presbyopia by providing improved intermediate and near visual acuity, while maintaining comparable distance visual acuity with a reduced need for eyeglasses, compared to a monofocal IOL. The AcrySof® IQ Vivity™ lens mitigates the effects of presbyopia by providing an extended depth of focus. Compared to an aspheric monofocal IOL, the lens provides improved intermediate and near visual acuity, while maintaining comparable distance visual acuity. All of these IOLs are intended for placement in the capsular bag.
WARNINGS/PRECAUTIONS:
General cautions for all AcrySof® and AcrySof® UV IOLs: Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting any IOL in a patient with any of the conditions described in the Directions for Use that accompany each IOL. Caution should be used prior to lens encapsulation to avoid lens decentration or dislocation. Physicians should target emmetropia, and ensure that IOL centration is achieved.
Additional Cautions associated with AcrySof® IQ ReSTOR® and AcrySof® IQ PanOptix® IOLs: Some patients may experience visual disturbances and/or discomfort due to multifocality, especially under dim light conditions. These may include some perceptions of halos or starbursts, as well as other visual symptoms. Therefore, patients implanted with multifocal IOLs should exercise caution when driving at night or in poor visibility conditions. A reduction in contrast sensitivity may occur in low light conditions. Spectacle independence rates vary with all multifocal IOLs; as such, some patients may need glasses when reading small print or looking at small objects. Patients should be advised that unexpected outcomes could lead to continued spectacle dependence or the need for secondary surgical intervention (e.g., intraocular lens replacement or repositioning). Posterior capsule opacification (PCO), may significantly affect the vision of patients with multifocal IOLs sooner in its progression than patients with monofocal IOLs.
Additional Cautions associated with AcrySof® IQ Vivity™ IOL: Most patients implanted with the AcrySof® IQ Vivity™ IOL are likely to experience significant loss of contrast sensitivity as compared to a monofocal IOL. Therefore, it is essential that prospective patients be fully informed of this risk before giving their consent for implantation of the AcrySof® IQ Vivity™ IOL. In addition, patients should be warned that they will need to exercise caution when engaging in activities that require good vision in dimly lit environments, such as driving at night or in poor visibility conditions, especially in the presence of oncoming traffic. It is possible to experience very bothersome visual disturbances, significant enough that the patient could request explant of the IOL. In the AcrySof® IQ Vivity™ IOL clinical study, 1% to 2% of AcrySof® IQ Vivity™ IOL patients reported very bothersome starbursts, halos, blurred vision, or dark area visual disturbances; however, no explants were reported.
Additional Cautions associated with AcrySof® IQ Toric, AcrySof® UV Toric ReSTOR® Toric, AcrySof® IQ PanOptix® Toric, and AcrySof® IQ Vivity™ Toric IOLs: Optical theory suggests that, high astigmatic patients (i.e. > 2.5 D) may experience spatial distortions. Possible toric IOL related factors may include residual cylindrical error or axis misalignments. Toric IOLs should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation. Prior to surgery, physicians should provide prospective patients with a copy of the appropriate Patient Information Brochure available from Alcon informing them of possible risks and benefits associated with the these IOLs.
ATTENTION: Refer to the Directions for Use labeling for the specific IOL for a complete list of indications, warnings and precautions.
ACRYSOF® IQ RESTOR® FAMILY OF MULTIFOCAL IOLS IMPORTANT PRODUCT INFORMATION
CAUTION: Federal (USA) law restricts this device to the sale by or on the order of a physician.
INDICATIONS: The AcrySof® IQ ReSTOR® Posterior Chamber Intraocular Multifocal IOLs include AcrySof® IQ ReSTOR® and AcrySof® ReSTOR® Toric and are intended for primary implantation for the visual correction of aphakia secondary to removal of a cataractous lens in adult patients with and without presbyopia, who desire near, intermediate and distance vision with increased spectacle independence. In addition, the AcrySof IQ ReSTOR Toric IOL is intended to correct pre-existing astigmatism. The lenses are intended to be placed in the capsular bag.
WARNINGS/PRECAUTIONS: Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting a lens in a patient with any of the conditions described in the Directions for Use labeling for each IOL. Physicians should target emmetropia, and ensure that IOL centration is achieved. Care should be taken to remove viscoelastic from the eye at the close of surgery.
The ReSTOR Toric IOL should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation.
Some patients may experience visual disturbances and/or discomfort due to multifocality, especially under dim light conditions. A reduction in contrast sensitivity may occur in low light conditions. Visual symptoms may be significant enough that the patient will request explant of the multifocal IOL. Spectacle independence rates vary; some patients may need glasses when reading small print or looking at small objects.
Posterior capsule opacification (PCO), when present, may develop earlier into clinically significant PCO with multifocal IOLs. Prior to surgery, physicians should provide prospective patients with a copy of the Patient Information Brochure available from Alcon informing them of possible risks and benefits associated with the AcrySof® IQ ReSTOR® IOLs.
Do not resterilize; do not store over 45° C; use only sterile irrigating solutions such as BSS® or BSS PLUS® Sterile Intraocular Irrigating Solutions.
ATTENTION: Reference the Directions for Use labeling for each IOL for a complete listing of indications, warnings and precautions.
Clareon® PanOptix® Family of Trifocal Hydrophobic IOLs
IMPORTANT PRODUCT INFORMATION
CAUTION: Federal (USA) law restricts this device to the sale by or on the order of a physician.
INDICATIONS
The Clareon® PanOptix® Family of Trifocal Hydrophobic IOLs include Clareon® PanOptix® and Clareon® PanOptix® Toric and are indicated for primary implantation in the capsular bag in the posterior chamber of the eye for the visual correction of aphakia in adult patients, with less than 1 diopter of pre-existing corneal astigmatism, in whom a cataractous lens has been removed. The lens mitigates the effects of presbyopia by providing improved intermediate and near visual acuity, while maintaining comparable distance visual acuity with a reduced need for eyeglasses, compared to a monofocal IOL. In addition, the Clareon® PanOptix® Toric Trifocal IOL is indicated for the reduction of residual refractive astigmatism.
WARNINGS/PRECAUTIONS: Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting a lens in a patient with any of the conditions described in the Directions for Use labeling. Physicians should target emmetropia, and ensure that IOL centration is achieved.
For the Clareon® PanOptix® Toric Trifocal IOLs, the lens should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation.
Some visual effects may be expected due to the superposition of focused and unfocused multiple images. These may include some perceptions of halos, radial lines around point sources of light (starbursts) under nighttime conditions, or glare, as well as other visual symptoms. As with other multifocal IOLs, there is a possibility that visual symptoms may be significant enough that the patient will request explant of the multifocal IOL. A reduction in contrast sensitivity as compared to that expected with a monofocal IOL may be experienced by some patients and may be more prevalent in low lighting conditions. Therefore, patients implanted with multifocal IOLs should exercise caution when driving at night or in poor visibility conditions.
Patients should be advised that unexpected outcomes could lead to continued spectacle dependence or the need for secondary surgical intervention (e.g., intraocular lens replacement or repositioning).
As with other multifocal IOLs, patients may need glasses when reading small print or looking at small objects. Posterior capsule opacification (PCO), may significantly affect the vision of patients with multifocal IOLs sooner in its progression than patients with monofocal IOLs. Prior to surgery, physicians should provide prospective patients with a copy of the Patient Information Brochure available from Alcon informing them of possible risks and benefits associated with the IOLs.
ATTENTION: Reference the Directions for Use labeling for each IOL for a complete listing of indications, warnings and precautions.
Clareon® Vivity™ Family of Extended Vision IOLs
IMPORTANT PRODUCT INFORMATION
CAUTION: Federal (USA) law restricts this device to the sale by or on the order of a physician.
INDICATIONS
The Clareon® Vivity™ Extended Vision Hydrophobic Posterior Chamber IOLs include Clareon® Vivity™ and Clareon® Vivity™ Toric IOLs and are indicated for primary implantation for the visual correction of aphakia in adult patients with < 1.00 D of preoperative corneal astigmatism, in whom a cataractous lens has been removed by extracapsular cataract extraction. The lens mitigates the effects of presbyopia by providing an extended depth of focus. Compared to an aspheric monofocal IOL, the lens provides improved intermediate and near visual acuity, while maintaining comparable distance visual acuity. The Clareon® Vivity™ IOL is intended for capsular bag placement only. In addition, the Clareon® Vivity™ Toric IOL is indicated for the reduction of residual refractive astigmatism in adult patients with pre-existing corneal astigmatism.
WARNINGS/PRECAUTIONS: Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting a lens in a patient with any of the conditions described in the Directions for Use labeling.
This lens should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation.
Most patients implanted with the Clareon® Vivity™ IOL are likely to experience significant loss of contrast sensitivity as compared to a monofocal IOL. Therefore, it is essential that prospective patients be fully informed of this risk before giving their consent for implantation of the Clareon® Vivity™ IOL. In addition, patients should be warned that they will need to exercise caution when engaging in activities that require good vision in dimly lit environments, such as driving at night or in poor visibility conditions, especially in the presence of oncoming traffic.
It is possible to experience very bothersome visual disturbances, significant enough that the patient could request explant of the IOL. In the parent AcrySof® IQ Vivity™ IOL clinical study, 1% to 2% of AcrySof® IQ Vivity™ IOL patients reported very bothersome starbursts, halos, blurred vision, or dark area visual disturbances; however, no explants were reported.
Prior to surgery, physicians should provide prospective patients with a copy of the Patient Information Brochure available from Alcon informing them of possible risks and benefits associated with the Clareon® Vivity™ IOLs.
ATTENTION: Reference the Directions for Use labeling for each IOL for a complete listing of indications, warnings and precautions.
Important Product Information – Clareon® Aspheric Hydrophobic Acrylic IOL with the AutonoMe® Automated Pre-loaded Delivery System
CAUTION: Federal law restricts this device to sale by or on the order of a physician.
INDICATION: The Clareon® Aspheric Hydrophobic Acrylic Intraocular Lens (IOL) is indicated for primary implantation in the capsular bag in the posterior chamber of the eye for the visual correction of aphakia in adult patients in whom a cataractous lens has been removed.
WARNINGS/PRECAUTIONS: The Clareon® IOL is intended for implantation in the capsular bag only. Physicians considering lens implantation under any of the following circumstances should weigh the potential risk/benefit ratio: Patients in whom the posterior capsule is ruptured, zonules are damaged, or primary posterior capsulotomy is planned.
DO NOT re-sterilize the Clareon® IOL or the AutonoMe® Delivery System by any method. DO NOT implant the IOL if the sterility has been compromised or if the sterile package has been unintentionally opened before use. DO NOT reuse the Clareon® IOL or AutonoMe® Delivery System. The device is for single use only.
The safety and effectiveness of the Clareon® IOL has not been substantiated in clinical trials in patients with certain pre-existing conditions and/or intraoperative conditions (listed in Tables 4 and 5 of the Directions for Use).
As with any surgical procedure, there is risk involved. Potential complications accompanying cataract and/or IOL implantation surgery may include, but are not limited to, the following: lens epithelial cell ongrowth, corneal endothelial cell damage, infection (endophthalmitis), toxic anterior segment syndrome (TASS), retinal detachment, vitritis, cystoid macular edema, corneal edema, pupillary block, cyclitic membrane, iris prolapse, hypopyon, anterior uveitis, hyphema, pigment dispersion, posterior capsule opacification, transient or persistent glaucoma, and secondary surgical interventions. Secondary surgical interventions include, but are not limited to: lens repositioning, lens replacement, vitreous aspiration or iridectomy for pupillary block, wound leak repair, and retinal detachment repair.
ATTENTION: Refer to the Directions for Use labeling for a complete list of indications, warnings and precautions.
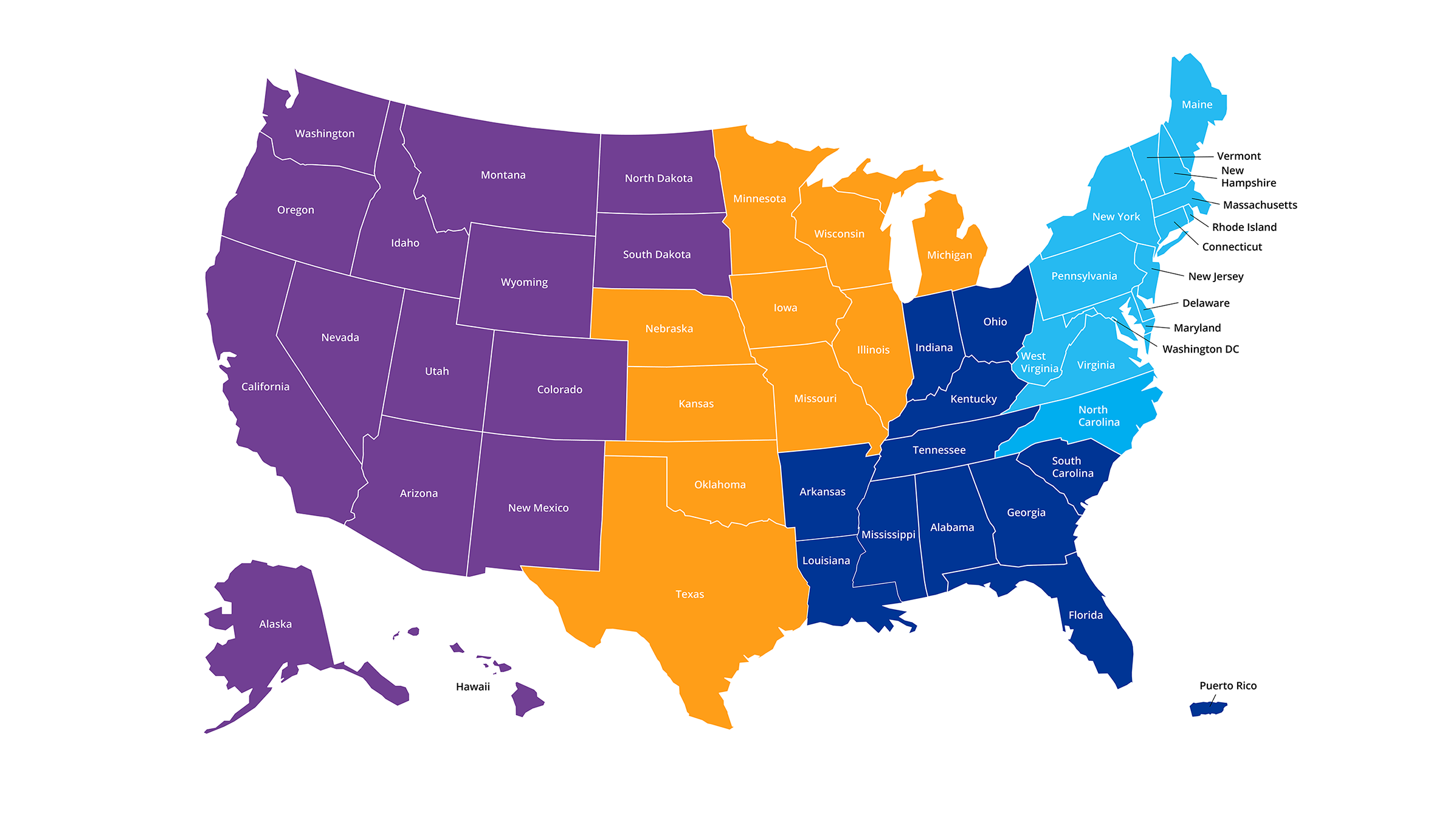
An Experienced Team Is Available To
Support Coverage, Coding And Billing
Questions In Your Local Area

Value Tool Resources
John Berdahl, MD
PanOptix® Cost-Benefit Analysis
Quality of Life Improvements
and Net Monetary Benefits for Patients
Cathleen M. McCabe, MD
Vivity™ Cost-Benefit Analysis
Quality of Life Improvements and Cost Savings for Patients.
Contact MARS
Please call our support line at: (866) 457-0277
Or click below to email us.